 While I've always had an interest in photography, both macro and micro, inside and outside the laboratory setting, my talent has never measured up to my enthusiasm. Regardless I continue to try my best to document interesting specimens that I, or my colleagues encounter in the microbiology laboratory.
While I've always had an interest in photography, both macro and micro, inside and outside the laboratory setting, my talent has never measured up to my enthusiasm. Regardless I continue to try my best to document interesting specimens that I, or my colleagues encounter in the microbiology laboratory.The photographs previous to this post were taken in entirety with a Leitz film camera head (right) mounted on the dual head Leitz microscope pictured below - all prior to the advent of digital imaging. Only the Tinea infected hair photo was taken using the same film camera mounted on a fluorescent microscope. Both Kodak Kodachrome & Ektachrome film of various
 speeds were previously employed.
speeds were previously employed.In 2006 I suffered an injury which required prolonged rehabilitation and equally long absence from the microbiology laboratory. I started this blog out of boredom and posted older photos which I had on hand. On my return I found the lab had retired the old film camera and replaced it with a Nikon Coolpix 8400. The versatility of digital imaging was immediately realized particularly in allowing numerous shots with no waste of costly film & processing and instant feedback as to whether a particular feature of interest was adequately captured.
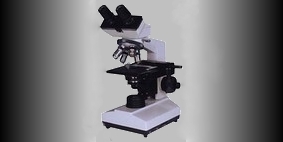
 Above is the dual head Leitz microscope with Nikon Coolpix 8400 thatI've used for the majority of photos that follow in this blog. Prior to the lab's aquisition of the Nikon, this same microscope was outfitted with a Leitz film camera pictured top above right.
Above is the dual head Leitz microscope with Nikon Coolpix 8400 thatI've used for the majority of photos that follow in this blog. Prior to the lab's aquisition of the Nikon, this same microscope was outfitted with a Leitz film camera pictured top above right.Microphotographs were fairly straight forward. The auto setting proved to be quite adequate for most stains and specimens. Flash was turned off. Photographs were captured in 300 dpi resolution and could be preserved or reduced in digital size or resolution as necessary. White balance could be corrected with photo editing software afterwords and cropped to focus attention on desired elements. Enhancements were kept to a minimum.
I quickly encountered several challenges when attempting macro photographs using the same Nikon 8400 in the laboratory setting, particularly in close-up photos of culture plates and media.
- Photos taken at close range could not be taken with a hand-held camera as it could not be held sufficiently steady so as not to blur the photograph. A tripod was found to be too cumbersome as it was always a challenge to set it to the appropriate height on a bench top without having the legs splay out too far. Tripod legs set closer together often became included in the critical area of the photograph and were difficult to crop out. A tripod became all but impossible to set up within a biological safety cabinet when photographing fungal cultures.
- Harsh overhead fluorescent lighting often produced both shadows and reflections from bench surfaces and clear plastic plate lids, frequently washing out the intended target.
Modifying the spring-loaded articulated arm of an unneeded desk lamp, I constructed a mechanism that joined the lamp arm to the screw mount base of a spare tripod. The arm can be mounted using a clamp base attached to a workbench or when used in a biological cabinet, it is mounted in an alternate heavy movable metal lamp base. The spring-loaded arm can be swung into place without the base showing in the photo. The springs allow easy height adjustment, bearing the weight of the camera as it is raised or lowered in conjunction with the camera's zoom in order to obtain the ultimate combination. While the springs are too sensitive to motion to allow one to depress the trigger by hand, the Nikon 8400 comes with an infra-red remote controller. Once the camera is positioned, the spring-loaded suspending arm is released and vibration allowed to stabilize (just a couple of seconds) before snapping the photo. The movable base allows the arm to be moved a location in the lab with the best available lighting. The background can be selected to best contrast the item being photographed. All fungal plate photography is carried out in the biological cabinet so as not to contaminate the lab with fungal spores.
 Above is my improvised spring-loaded lamp base with attached tripod screw mount allowing the camera position to be altered without getting the base into the photo. The heavy movable base allow the camera to be set up in a laminar flow hood for the safe photography of fungal cultures. A matt-black folder cover provides a disposable, non-reflective contrasting background for clear plates such as SAB. I rarely use the camera's flash as it tends to wash out images at the distances I require and reflections are hard to control.
Above is my improvised spring-loaded lamp base with attached tripod screw mount allowing the camera position to be altered without getting the base into the photo. The heavy movable base allow the camera to be set up in a laminar flow hood for the safe photography of fungal cultures. A matt-black folder cover provides a disposable, non-reflective contrasting background for clear plates such as SAB. I rarely use the camera's flash as it tends to wash out images at the distances I require and reflections are hard to control.New Toys (2010);
In the fall of 2010, our lab purchased a Leitz DMD-108 digital microscope. This headless (no occular) microscope utilizes LED illumination with the digital image being transmitted to a high-definition monitor. This unit allows for;
- immediate monitoring of images by multiple observers displayed real time on the high-definition screen.
- photography of any interesting field captured to a jump drive or other mountable storage medium.
- record of camera/exposure settings for each image captured.
- dictation of description of image features captured with built in microphone.
- real time transmission of image over internet for consultation.
- X & Y co-ordinates of image location for future orientation.
- Digital zoom in addition to magnification provided by standard objectives.
- White balance adjustment & colour correction for various common stains or custom settings.
While an interesting instrument, it's usefulness in a microbiological setting is yet to be determined. The instrument seems better suited for histological/pathological specimens.
 Above is a LPCB fungal specimen as seen on the high-definition monitor projected from the Leitz DMD-108 microscope seen on the right of the photo. Note the lack of occulars on this microscope.
Above is a LPCB fungal specimen as seen on the high-definition monitor projected from the Leitz DMD-108 microscope seen on the right of the photo. Note the lack of occulars on this microscope.In the future I will note any photos taken on this microscope. All others will continue to be taken using the Nikon 8400.
For anyone wishing to know more about the Leica DMD-108 Digital Imaging Microscope you can visit their site for specific information.

.jpg)























