An earlier post showed how to make a Slide Culture of a Fungus for examination. In this post I'll show how to make an adhesive tape preparation of a Fungus for examination.
Adhesive Tape Preparation or Slide Culture - which should I make? Well, try both if you can. I find that sometimes one may be superior to the other when trying to capture the structure of a particular fungus.
The adhesive (sticky) tape preparation pulls up the structures and sort of locks them in place -stuck to the tape. Or, it may be a bit more disruptive and perhaps destroy or scatter some of the fungal features. You never no for sure until you try. The other issue is the clarity of the tape itself. Adhesive tape has the glue laid down on one side to make it sticky. The evenness of the glue as well as the transparency of the tape can affect the quality of the image when viewed. Adhesive tape is manufactured specifically for use with mycological specimens however I've found that any good commercial adhesive tape, such as Scotch Tape™, is quite adequate. It should be obvious but I'll say it anyways: use only clear tape -do not use 'frosted' tape!
The advantage of a slide culture is that the fungus can attach itself to the coverslip as it grows and if removed very carefully, the features will not be disturbed and can be viewed as they naturally occur. The trick is to be very gentle when removing and mounting the cover slip.
Well, nothing could be simpler: Just grow your fungus on appropriate media and take a sample. Structures may develop over time so you may wish to make adhesive tape preparations of the same fungal colony over several days. Structures may also deteriorate or disappear on prolonged incubation so timing is important in making both adhesive tape preparations and slide cultures.
Important!!! Make all preparations within a biological safety cabinet (BSC) rated at a Level 2.
Here we go:
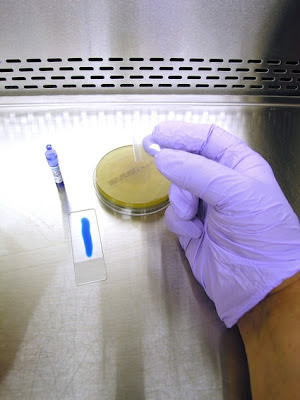
1. Get your supplies together. You will need some adhesive tape (1). Pictured here is a roll of 'Fungi-Tape manufactured by a laboratory supply company specifically for this purpose. Also shown is a roll of Scotch™ brand transparent tape which is just as good. You want to select a tape that is about the width of the slide. Of course you will need a microscope slide (2) and some Lactophenol Cotton Blue (LPCB). This can be made up in the lab (formula at the end of this post), or can be bought "ready to use" from laboratory supply companies. Pictured here is an ampule of LPCB (3) manufactured by Becton-Dickenson. The squeeze dropper is a quick and clean way of dispensing LPCB to the slide.
2. Dispense a line of LPCB on the slide. The amount will dispensed will come with practice. Too much and the tape will float with the LPCB oozing out the sides. Too little and any spores present will not come in contact with the LPCB, posing a possible contamination problem. I like to stick one end of the tape to my thumb and the other end to my middle finger. (admittedly rather difficult to see in this photo due to the cramped confines of the BSC.)
3. This allows me to use my free index finger (pointer finger) in between my thumb and middle finger to push the sticky side of the tape down onto the colony.
6. As you remove the tape from the fungal colony, a representative sample of the growth should remain stuck to your tape.
7. Line up and place the tape onto the slide where the LPCB should spread out to the edges of the tape. This is somewhat tricky. If you don`t manage to place the tape down exactly parallel to the edges of the slide, the tape will overhang the glass slide. Spores loosely adhering to the tape may present a contamination hazard and the tape may stick to the microscope stage compromising its movement. Removing and reapplying can be done but may further disturb the structures on the tape.
8. The adhesive tape preparation slide is ready to be viewed on a microscope (boy, I didn`t make a nice and even looking preparation for this photo, did I! - but you get the idea.) I`m both making the preparation and taking the photos so both hands are full.
With a bit of skill, you can also use sticky tape to sample a fungus growing in a test tube.
1. Wrap tape into a circle, sticking one end to the other and then sticking the loop (arrow) to a wooden applicator stick or other long thin item. Carefully insert the adhesive tape loop into the test tube culture media and press against the fungal growth. Pull the applicator stick with the tape out of the test tube. Going in and out takes patience and practice for if you touch the glass neck of the test tube with the tape, it will no doubt stick to the glass and your attempt will be ruined.
2. Once outside, carefully cut the tape loop open with scissors and place tape onto the microscope slide with LPCB.
Disinfect your scissors and dispose of all used materials in a safe manner!!
- Lactic Acid acts as a clearing agent and helps preserve the fungal structures
- Phenol kills the fungus making it safe to remove your slide preparation from you biological safety cabinet (BSC). Spores are often abundant and can easily infect the mycologist or contaminate the laboratory if not killed.
- Glycerol is slightly viscous and prevents drying of the prepared slide specimen.
- Cotton Blue is an aniline dye which adds colour to the fungal preparation thereby enhancing and contrasting the structures.











.jpg)























